Description
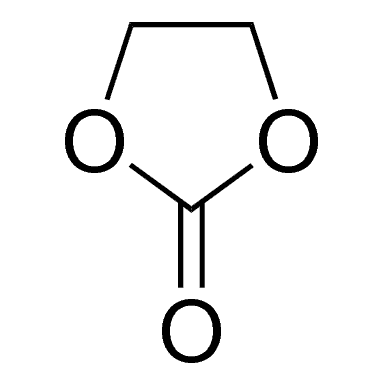
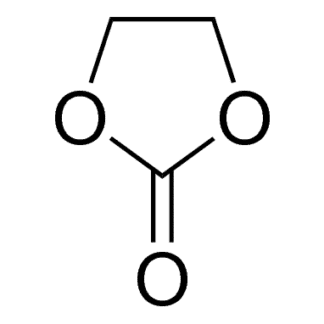
Ethylene carbonate is one of the most important solvent components in Lithium-ion Batteries (LIB) Electrolytes. It is the only organic solvent that enables the solid electrolyte interface (SEI) to be formed on the surface of graphitic carbons, which helps the graphite anode reversibly react with lithium ions for hundreds of cycles. In most of the LIB electrolytes, ethylene carbonate takes 20% ~ 35% in the mixed solvents and takes 15%~25% overall in the LIB electrolyte. More ethylene carbonate in the Electrolyte is helpful for the cycling of graphite anode while decreasing the low-temperature performance and rate capability due to its high viscosity and high melting point.
| Chemical Name | Ethylene Carbonate |
| CAS# | 96-49-1 |
| Molecular formula | C3H4O3 |
| Assay | >99.9% |
| Moisture | <100ppm |
| Acid | N/A |
| Molecular weight | 88.06 |
| Melting point | 35-38 degree C(lit.) |
| Boiling point | 1.321 g/mL at 25 degrees C(lit.) |
| Density | 1.321 g/mL at 25 degree C(lit.) |
| Flash point | 320 degree F |
| Form | Crystalline low melting solid |
| Color | Colorless |
Caution
This organic reagent is packaged in fluorinated HDPE bottles with ultra-low water content (less than 100 ppm). The bottle is tightly closed and sealed with an aluminum bag. Please handle it in an inert and moisture-free glove box or dry room. Gently warm the container to melt the solid. Avoid high-temperature heating or igniting. Store in a glove box or dry room.



Reviews
There are no reviews yet.